Second FDA approval in a few weeks: Momentis Surgical, a medical device company that has developed a tiny, mobile robotic system for a variety of surgeries that operates through only one incision, announced today that the US Food and Drug Administration (FDA) has granted 510(k) approval for the second generation of the company’s robotic surgery platform, Anova. This is the second FDA approval that Momentis has received in a few weeks, after the company announced in October the 510(k) approval for the first generation of Avono. The approval from October marks an expansion of the system’s capabilities to repair a hernia in the abdominal wall (ventral hernia) with one entry incision following the approval to perform gynecological surgical procedures with a transvaginal approach.
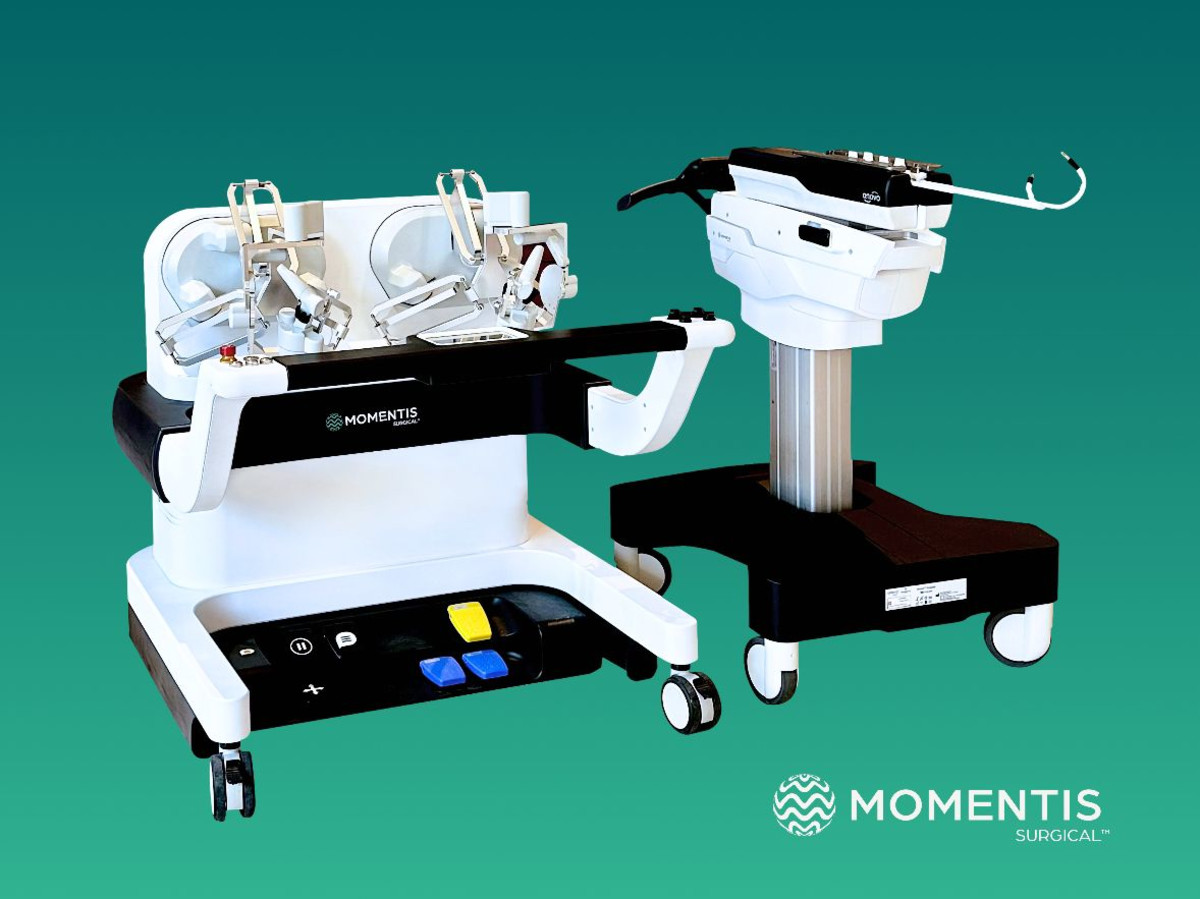
The second generation of the Anovo platform, based on many years of clinical insights, offers a suite of upgrades that increase the surgeon’s experience and ease of use. This is the first robotic system to receive FDA approval (single port or multiple port) for surgery in both forward and backward bending positions and thus provides unprecedented access and use of joints through a single entry incision. Surgeons will now experience expanded ergonomic capabilities through sensory feedback that enables intuitive control of the robotic arms during surgical procedures. Furthermore, a video layer in which notes can be integrated strengthens the communication between the team of surgeons in the room and increases the cooperation and mutual learning between them.
Momentis Surgical received the first FDA approval for the Anovo system for a wide range of indications in women’s medicine, including surgeries in the abdominal cavity through the vagina, which reduce pain and the risk of infections and enable faster recovery than usual without leaving scars. This system is the first and only FDA-cleared for minimally invasive robotic surgery that has human arm-like robotic arms that provide human-level fine motility, with 360-degree rotatable joints and multi-plane flexibility. The surgical tools were designed to simulate the movements and capabilities of the surgeons’ arms, with shoulder, elbow and wrist joints. Multiple devices can be inserted into the body being operated on through a single entry incision, and the 360-degree rotation capability allows avoiding obstacles as well as optimal access and working angles.
Founded and managed by Dvir Cohen, Momentis Surgical is revolutionizing the field of robotic surgery by developing innovative and tiny robotic technologies for a growing number of medical applications. The company’s goal is to provide surgeons with tools to perform complex, minimally invasive surgeries that will enable better results for patients, reduce expenses and allow access to more surgeons, patients, hospitals and surgical centers around the world for surgical robotics. Momentis Surgical’s investors include leading investment funds and entities in the fields of life sciences, including: Peregrine Ventures, OurCrowd, Ceros and Accelmed.
Dvir Cohen, CEO and co-founder of Momentis Surgical, said: “The advancement of technology and the expansion of surgical indications emphasize our commitment to the long-term plan to bring about a revolution in the field of robotic surgery. The FDA approval is another milestone in the execution of our multi-year plan to take robotic surgeries to the next level. We are in a strong momentum following impressive results of clinical trials in gynecological surgeries. Alongside these improvements, we strive to expand our global presence and present Anovo in the European and Asian markets in the future.”
Eyal Lipshitz, Managing Partner, Peregrine Venture Capital Fund, which has accompanied Momentis Surgical since its inception, added, “We have been able to build in our incubator a large number of successful companies, among them Momentis, which has the potential to change the status quo in the field of robotic surgery. The Anovo system will expand access to surgery robotics for a greater number of surgeons in a greater number of medical institutions, including ambulatory surgical institutes. This is thanks to the small dimensions of the multidisciplinary system and its friendliness.”
Texas License Plate
texaslicenseplate Publisher Publications – Issuu
Certificate verification problem detected
Earthly Directory.com – Search Listings > Search Results
Utah License Plate | Posteezy
utahlicenseplate Publisher Publications – Issuu
Celestial Directory.com – Search Listings > Search Results
Vermont License Plate | Smore Newsletters
Vermont License Plate – cally.com
virginialicenseplate Publisher Publications – Issuu
Virginia License Plate (Business Opportunities – Other Business Ads)
Best Social Bookmarking Sites 2022
Lemon Directory .com : Washington License Plate
Best Social Bookmarking Sites 2022
Washington License Plate – JustPaste.it
Dark Scheme Directory.com – Search Listings > Search Results
Washington D.C. License Plate | Business Social Network | B2BCO
Washington D.C. License Plate – Business & Professional Services – Veterans and Patriots in Business
West Virginia License Plate, Business Service, 5707 MacCorkle Ave SE,
Certificate verification problem detected
WhoFish – Local Businesses
Certificate verification problem detected
Certificate verification problem detected
Wisconsin License Plate – Wakelet
Certificate verification problem detected