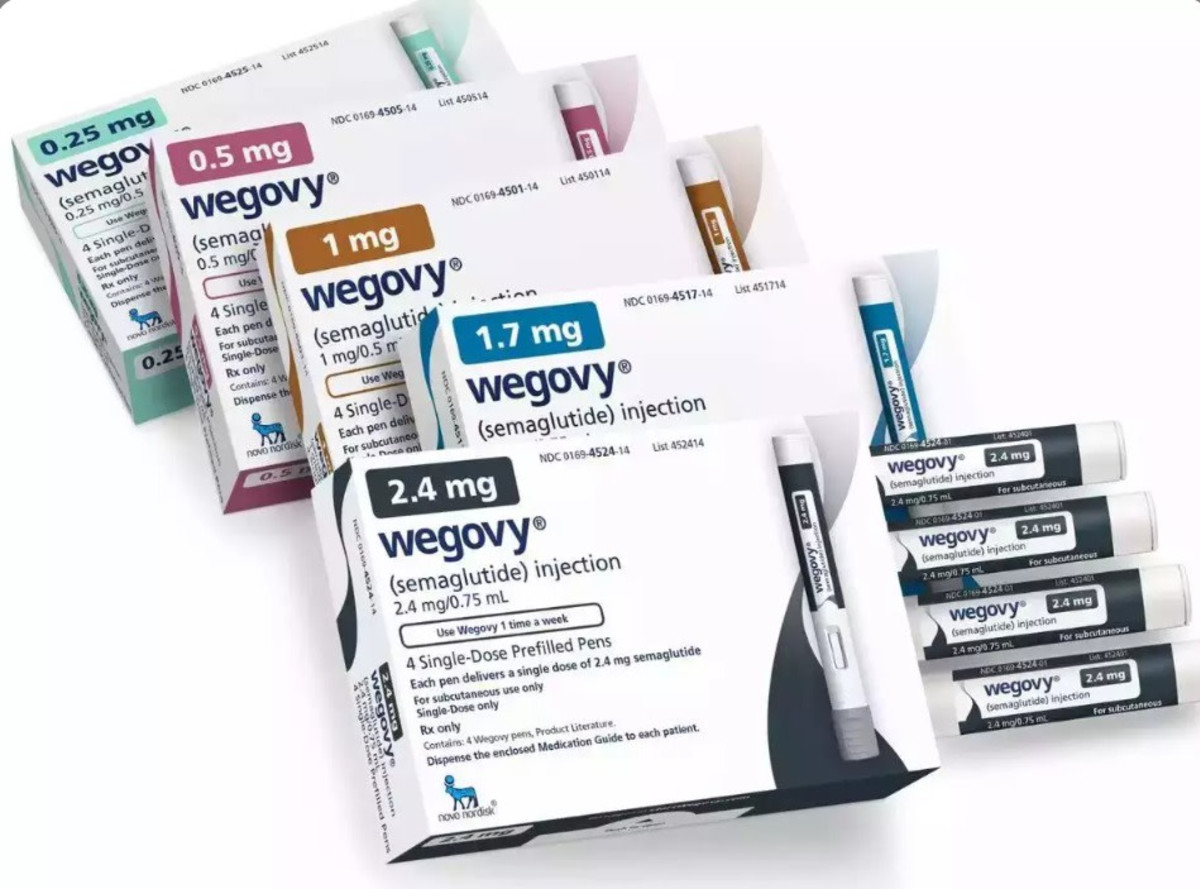
The Novo Nordisk company has announced that the European Union Commission has approved a higher dose of the weight loss injection Vigobi intended for the treatment of obesity. The approval comes after a positive recommendation by the European Medicines Regulator last December, and allows doctors throughout the Union to prescribe patients a dose of 7.2 milligrams per week.
According to the company’s announcement, the new dose will be given at this stage through three separate injections of 2.4 milligrams, taken consecutively at the same time, once a week. Meanwhile, the company said that it submitted an application for approval of a dedicated disposable pen with a dose of 7.2 milligrams. If approved, it could be available as early as this year.
The basis for approval rests on the results of an advanced phase study that lasted 72 weeks and included 1,407 obese or overweight adults without diabetes. The participants who received the 7.2 milligram dose lost an average of 20.7% of their body weight, compared to an average decrease of 17.5% among those who received the standard dose of 2.4 milligrams. The difference between the groups is considered statistically significant and indicates the potential for further weight loss among some patients.
Vigobi is based on the active substance semaglutide, an analogue of the GLP 1 hormone produced naturally in the intestine. This hormone is secreted after eating and affects the satiety centers in the brain, mainly in the hypothalamus, slowing down the emptying of the stomach and reducing the feeling of hunger. The drug mimics the action of the hormone, binds to the designated receptors and increases the feeling of satiety over time. The result is a decrease in calorie consumption and gradual weight loss.
The treatment is given by subcutaneous injection once a week, and is intended for adults with a body mass index of 30 or higher, or 27 or higher in the presence of concomitant diseases such as hypertension, dyslipidemia or sleep apnea. The common side effects include nausea, vomiting, diarrhea and constipation, especially in the stages of starting treatment and increasing the dose. In rare cases, pancreatitis or gallbladder disorders have been reported, so regular medical follow-up is required.
In recent years, Vigobi has become one of the most sought-after drugs in the field of obesity treatment, against the backdrop of a sharp increase in obesity rates worldwide and a growing recognition of obesity as a chronic disease that requires ongoing treatment. On the other hand, experts emphasize that the drug is not a substitute for a lifestyle change, and its maximum effectiveness is achieved by combining a balanced diet and physical activity.
The approval for the higher dose may expand the possibilities of personal adjustment of the treatment, especially for patients who have not achieved sufficient weight loss with the existing dose. However, it is not yet clear how the new dose will be priced and whether the public health systems in the EU countries will finance it fully or partially.
The European move comes against the background of increasing competition in the obesity drug market, with additional developments from the GLP 1 family and combined substances that work on additional hormonal mechanisms. At this time, the new Vigobi dose is one of the highest levels of semaglutide approved for the treatment of obesity in Europe.
Recently, an oral version of semaglutide was also approved in Europe for the treatment of obesity, given as a daily pill instead of a weekly injection. It is actually a development based on the same mechanism of action of Vigobi, but given orally. In clinical studies that lasted about a year, an average decrease of about 15% of body weight was demonstrated, depending on the dose and the characteristics of the patients, a figure slightly lower than the decrease observed with the high doses of the weekly injection. The pill is already marketed in several European countries, and in some markets outside of Europe it is available under different trade names based on semaglutide, while a wider launch is expected later this year subject to the approval of the local ministries of health.
https://hedgedoc.info.uqam.ca/s/jCONsITP7
https://rentry.co/mke33tct
http://catholicquestions.ca.wiringdoneright.com/index.php?qa=user&qa_1=levelcake3
https://notes.netd.cs.tu-dresden.de/s/e6-iMUMaJ
https://paripesa-chile.com/deportes-electronicos/cs2/
https://irte.duiko.guru/forums/users/trowelpizza43/
https://partecipo.prato.it/profiles/empresaendoterapiave/badges
https://www.pinterest.com/pin/1133992381200172457
https://www.trustpilot.com/review/urooncoconnect.com
https://pinco-canada.net/privacy-policy/
https://hedgedoc.stusta.de/s/Ss4c6qTAd
https://eso-community.net/memberlist.php?mode=viewprofile&u=22464
https://dreevoo.com/profile.php?pid=1082547
https://pinco-canada.net/fr-ca/bonus/
https://trueluck-global.com/en-ie/mma/
https://tutos.cemea.org/s/7nTo5aoEC
https://pinco-ca.net/mma/
https://batery-aviator.in.net/real-or-fake/
https://www.tumblr.com/sienblog/808041352441085952/la-veloce-crescita-della-potenzialit%C3%A0-di-energia
https://solo.to/dondecenarentorre
https://molchanovonews.ru/user/cityjump90/
https://jbcasinobe.com/android/
https://1xbet-costarika.com/tenis/
https://diego-maradona.org/user/animalcuban94/
https://pixabay.com/es/users/54548148/