The American Food and Drug Administration (FDA) granted 510(k) approval to Momentis Surgical’s Anovo robotic surgery platform for ventral hernia repair. This approval is added to the company’s existing FDA approvals for performing gynecological surgical procedures with a transvaginal approach.
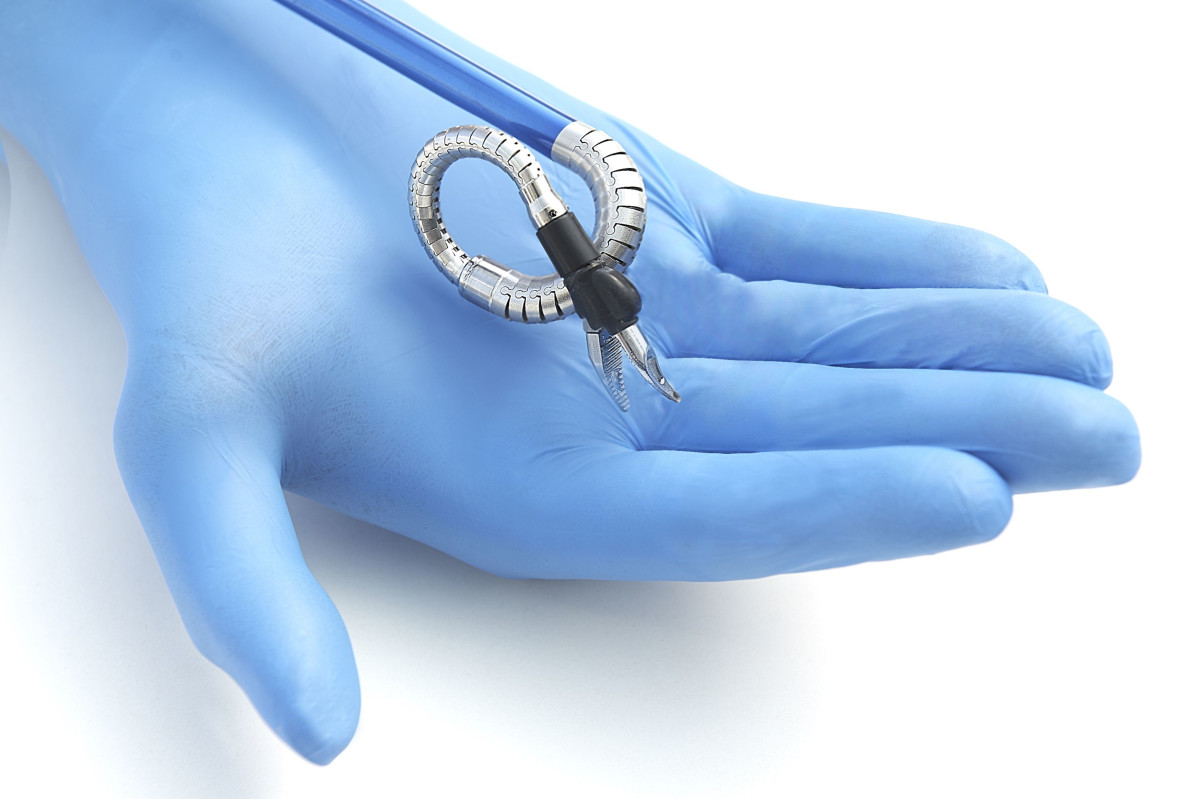
Momentis Surgical has developed a tiny and mobile robotic system for a variety of surgeries that operates through only one entrance incision. Anovo is the world’s only FDA-cleared minimally invasive robotic surgery platform for single-incision abdominal hernia repair. The system is characterized by robotic arms resembling a human arm, with joints that can rotate 360 degrees and are flexible in several planes. Several devices can be inserted into the operated body through a single entry incision.
Momentis Surgical develops innovative and tiny robotic technologies for a growing number of medical applications, with the goal of improving surgical outcomes, reducing expenses and increasing accessibility to surgical robotics.
Dvir Cohen, CEO and co-founder of Momentis, said: “We leveraged the clinical success of the Anovo platform in gynecology to expand into general surgery. Thanks to its size and portability, the system is also suitable for hospitals as well as doctors’ clinics.”
Eyal Lifshitz, managing partner at the Peregrine Venture Capital Fund, added: “Momentis has made significant progress in the past year to expand the clinical capability of its robotic system.”
Prof. Yoav Mintz from the Hadassah Medical Center said: “The Momentis robot offers a unique approach to robotic surgery. The flexible arms along with a single penetration point allow for improved maneuverability along with a less invasive approach.”
Dr. Frederic H. Moll, a member of the Momentis board of directors, added: “The company has now created a true robotic platform, and therefore it is a unique technology that will have an immediate impact on the way general surgery is conducted.”