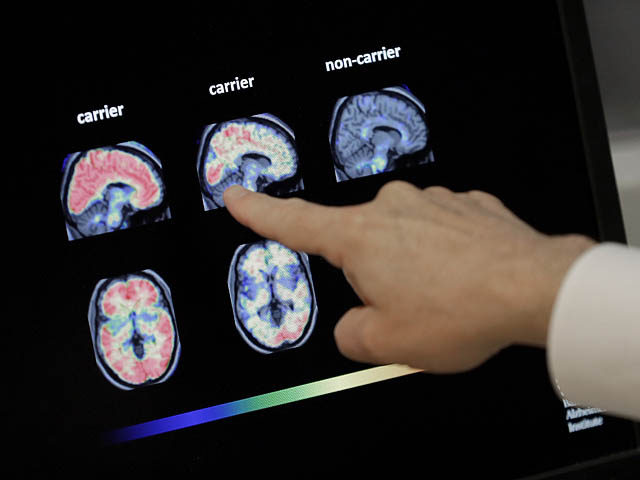
The Food and Drug Administration (FDA) has approved Eli Lilly’s drug donanemab, which slows the progression of Alzheimer’s.
Eli Lilly said the drug would be sold in the United States under the brand name Kisunla and is intended for adults with early symptoms of Alzheimer’s disease.
Donanemab is given as a monthly infusion. A six-month course will cost about $12,500.
In a study, a drug that targets amyloid plaques in the brain was shown to slow the progression of Alzheimer’s disease by 35% over 18 months compared to a placebo.
Radio Kan Bet reported that the new Eli Lilly drug is under review by the Israeli Health Ministry and is expected to be included in Israel’s “drug basket” in 2025 or 2026.